Introduction: Molecular classifications have been developed to characterize the heterogeneity of diffuse large B cell lymphoma (DLBCL), with activation of BCL6 and Notch signaling pathways acting as driving pathogenic features in C1/BN2 subtype tumors. Among C1/BN2 defining variants are truncating/inactivating SPEN mutations. SPEN regulates the Notch signaling pathway by initiating formation of a repressive complex that facilitates transcriptional repression of Notch target genes. Our group has identified SPEN mutations enriched in DLBCL cases failing to achieve event-free status at 24 months (EFS24). This contrasted with generally favorable outcomes in C1/BN2 tumors, suggesting a potential high-risk molecular feature with unexplored significance. Presenting an opportunity to expand underlying mechanisms and risk stratification in C1/BN2 tumors, we aimed to use a functional genomic approach, combined with clinical data, to characterize the alternative biology stemming from SPEN truncating mutations.
Methods: FFPE tumor biopsies from newly diagnosed DLBCL cases (N=444) enrolled in the Mayo Clinic/Iowa Lymphoma Molecular Epidemiology Resource (MER) program were analyzed by WES (n=404) and RNA-seq (n=321). Biologic and clinical variables were annotated for all cases. CRISPR/Cas9 introduced truncating mutations to the endogenous SPEN locus in OCI-LY3 & SU-DHL-2 ABC-type human cell lines to establish models of SPEN depletion. Functional in vitro analysis utilized standard methods for RNA-seq, cell proliferation, western blot, and flow cytometry.
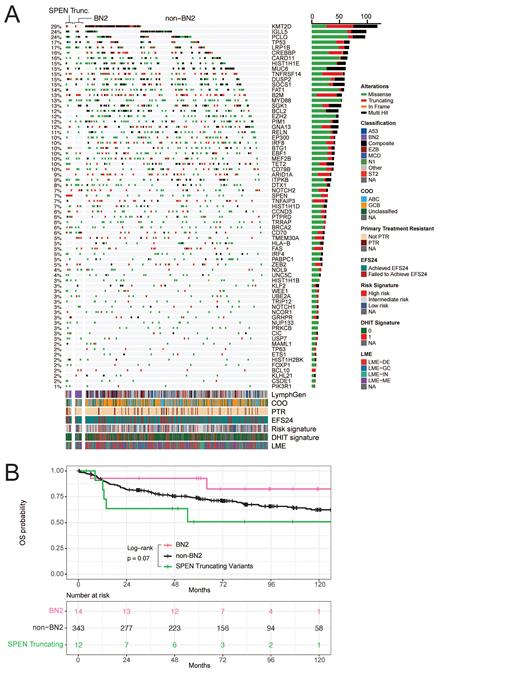
Results: WES identified 29/404 ndDLBCL (7.2%) harboring SPEN mutations; n=17 missense and n=12 nonsense or frameshift insertions/deletions (truncating variants - [trunc]), the later considered as the focal point of our study. Against all cases, SPEN trunc cases displayed enrichment for high-risk factors including non-GCB COO (p=0.06, OR=3.7, 95% CI [0.9, 22.2]) and primary refractory disease (PTR; p=0.02, OR=5.0, 95% CI [1.05, 19.9]), and demonstrated inferior OS (p=0.06, HR=2.2), where a majority of clinical progression events occurred within 12 months of diagnosis. Leveraging RNA-seq, we observed 11 of 11 SPEN trunc cases exhibiting a double-hit negative gene expression signature, 9/11 cases with inflammatory or depleted microenvironment (LME), and 8/11 with a signature associating with intermediate/high risk of EFS24 failure. Applying the LymphGen algorithm, 5/12 SPEN trunc cases received the BN2 classification, observing co-occurring mutations with CD70 (p<0.01, OR=9.68), BRCA2 (p=0.03, OR=5.84), TET2 (p=0.02, OR=4.91), IRF4 (p=0.02, OR=7.27), and PTPRD (p<0.01, OR=8.31) genes, and were mutually exclusive with ARID1A, BCL2, EZH2, FAS, HLA-B, IRF8, and TMEM30A mutations ( Figure 1A). Isolating SPEN trunc against BN2 cases without SPEN variants (n=14), despite similar clustering of non-GCB COO (p=0.7), PTR was exclusively observed in SPEN trunc cases, accompanied again by inferior OS (p=0.07, HR=4.2; Figure 1B). In the OCI-LY3 SPEN depletion model, GSEA analysis from RNA-seq revealed activation of E2F (NES=2.7; p=9.4E-20) and MYC target genes (NES=2.3; p=4.1E-6), while both OCI-LY3 and SU-DHL-2 models featured depletion of antigen presentation genes (NES= -1.8, p=5.7E-4; NES= -2.2, p=3.8E-7, respectively). A decrease of MHC class II proteins (HLA-DR, HLA-DP) was confirmed by flow cytometry in both models (p<0.01). Hypothesizing loss of SPEN enhances reliance on the BCL6-HDAC3 axis featured in BN2 tumors, we treated both SPEN depletion models with a class-I HDAC inhibitor highly selective for HDAC3, BRD-3308, and indeed observed a 1.4 and 2.4-fold increase in sensitivity compared to WT cells (p=0.07, p<0.01, respectively).
Significance: We find SPEN trunc mutations in poor acting DLBCL cases with clinical and biologic characteristics that diverge from C1/BN2 biology. The enhanced rate of clinical progression highlights urgency to better characterize these tumors. Our data suggests loss of SPEN regulatory mechanisms may act cooperatively with C1/BN2 biology to facilitate tumor immune evasion and drive progression through Notch-mediated pathways. Importantly, in vitro evidence shows SPEN deficient lymphoma cells are susceptible to targeted therapeutics against dependent pathways, providing rationale for advancing individualized care strategies for affected patients.
Disclosures
Wenzl:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Maurer:BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Rimsza:Roche: Other: Consulting; NanoString: Other: Licensed intellectual property. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Ansell:Pfizer, Inc: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; Affirmed: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research. Witzig:ADC: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Research Funding; Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding. Nowakowski:MEI Pharma: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy; Selvita Inc: Consultancy; F Hoffmann-La Roche Limited: Consultancy; TG Therapeutics: Consultancy; Debiopharm: Consultancy; Zai Lab Limited: Consultancy; Kymera Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy; Abbvie: Consultancy; Curis: Consultancy; Seagen: Consultancy; Celgene Corporation: Consultancy; Blueprint Medicines: Consultancy; Bantam Pharmaceutical LLC: Consultancy; ADC Therapeutics: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cerhan:Protagonist: Other: Safety Monitoring Committee; Genmab: Research Funding; NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding. Novak:Bristol Myers Squibb: Research Funding.